Bitan Research Lab
About our lab
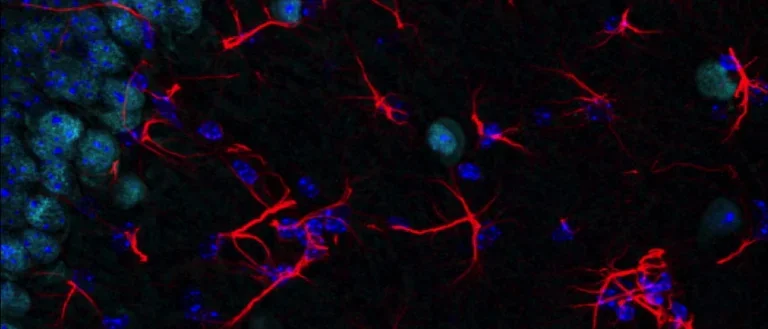
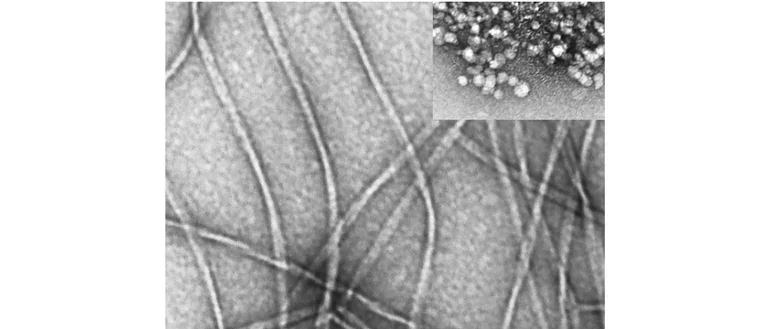
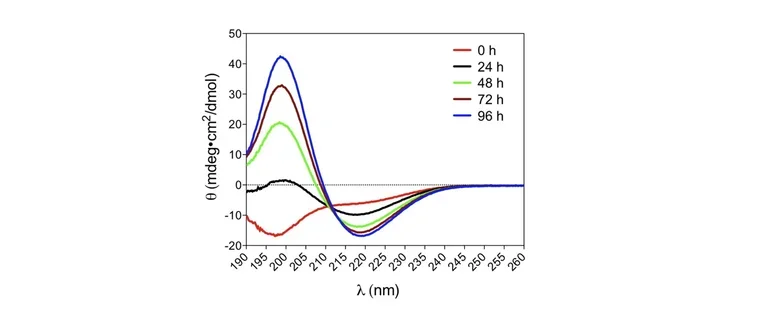
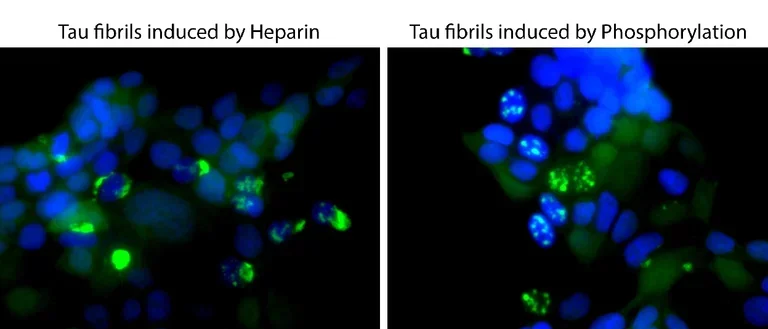
Our group studies neurodegenerative diseases involving abnormal self-association of proteins into toxic oligomers, aggregates, and amyloid fibrils. We study the molecular interactions in these processes and how they spread through the brain. We also develop novel biomarkers and drug candidates for these diseases.
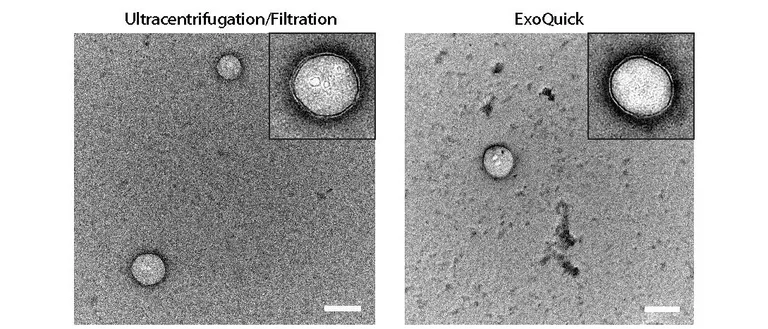
Abnormal protein oligomerization and aggregation cause, or are involved in, over 50 diseases called amyloidoses or proteinopathies. Of this large family of diseases, we study Alzheimer’s disease, Parkinson’s disease, and other dementias and movement disorders. An important focus of our lab is the analysis of biomarkers that can improve the prognosis and diagnosis of these diseases and facilitate drug development for them.
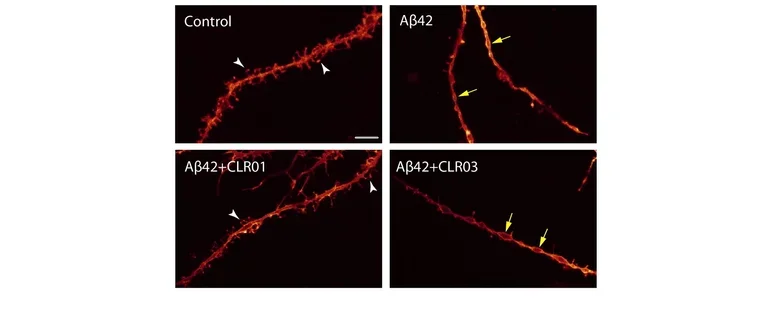
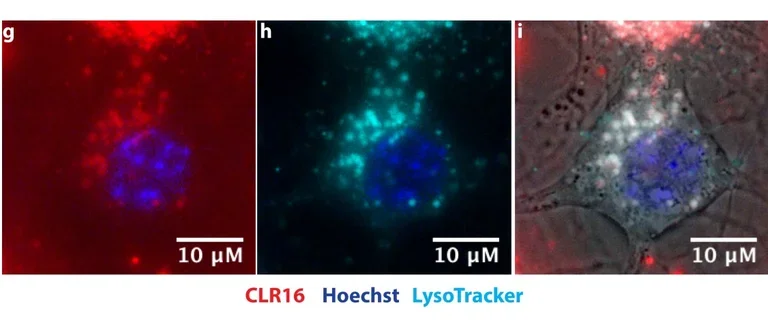
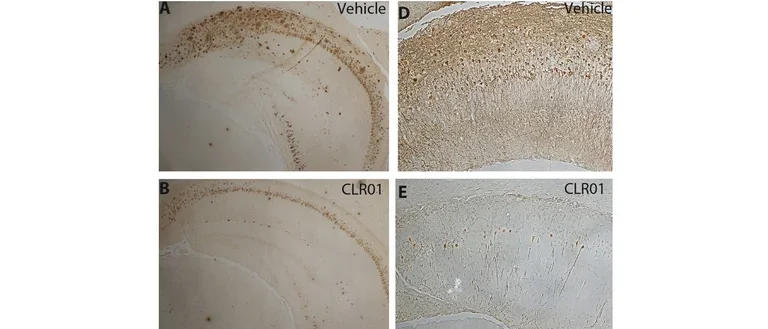
Another major project in our lab is the development of “Molecular Tweezers” as novel drug candidates for proteinopathies.


Gal Bitan, Ph.D
Professor of Neurology
David Geffen School of Medicine at UCLA
Dr. Bitan is affiliated with three graduate programs:
- Biochemistry, Biophysics & Structural Biology (BBSB)
- Interdepartmental PhD Program in Neuroscience (NSIDP)
- Molecular & Medical Pharmacology
The Bitan Lab logo was designed by former lab member Otmane Lahgui, MS, MBA.